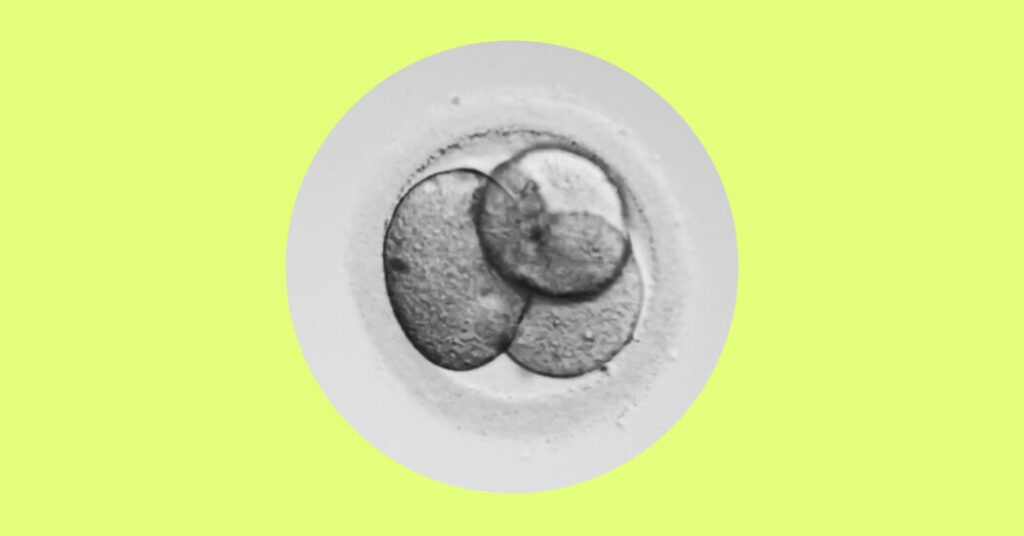
On June 24, 2022, the same day the Supreme Court issued its decision in Dobbs v. Jackson Women’s Health Organization, I received a call from the fertility clinic where I’d been undergoing in vitro fertilization, informing me that seven of my fertilized eggs had made it to the five-day-old blastocyst stage.
The next morning, I went to the clinic, lay down on a table and watched on a black-and-white screen as one of the seven — the one the embryologist had deemed the most likely to develop into a child — was transferred into my uterus. It was an unsettling moment to find myself responsible for half a dozen embryos. The Supreme Court’s ruling was, first and foremost, about abortion. But it was also, explicitly, a statement about the importance of “potential life,” a phrase that appears repeatedly in the opinion. Such a ruling in our political environment would surely set in motion some unexpected and, in some cases, unintended consequences.
An embryo, in medical terms, is a fertilized egg through the eighth week after fertilization. At that point, the tiny ball of cells is no bigger than a raspberry, but its impact on the body can be mighty. Within a few weeks of the transfer, I experienced the first wash of nausea. I saw blood when I went to the bathroom and feared that it — whatever it was at that stage — was gone. But it decided to stay, and as the pregnancy progressed, I read headlines about a Texas woman justifying driving in the H.O.V. lane because she was pregnant and a tax break in Georgia for unborn children.
In the years since Dobbs, state after state banned abortions, and referendum after referendum enshrined abortion rights. Fertility patients in states like Texas and Tennessee worried about the legal status of their treatments, as anti-abortion politicians insisted that life began at conception. In February 2024 an Alabama court ruled that frozen embryos in an I.V.F. clinic were children, their destruction valid grounds for a wrongful-death claim. Until this decision, attempts to cement the links between abortion and embryonic personhood had largely ignored I.V.F., since it is widely perceived as a tool to create, rather than destroy, “potential life.” But this was a blunt assertion of full legal personhood for these little bundles of possibility that exist, as one scholar has put it, “at the borders of science, morality and democracy.”
Since then, arguments over abortion have become more contested and more tightly entwined with the status of embryos, even outside their more familiar place inside the womb. Neither of these developments will help us, as a nation, grapple with the increasingly urgent questions about how we ought to treat them.
When Louise Brown, the world’s first baby to be conceived via in vitro fertilization, was born, there were no rules governing the new technology.
Keystone/Getty Images
Starting in 1978, when the first I.V.F. baby was born, embryos have been appearing outside the womb more and more often. Today, hundreds of thousands of them are banked away, sometimes abandoned, in cryogenic storage tanks. (Exactly how many frozen embryos exist in the United States is unclear, since there is no requirement to record their number, unlike in other countries.) They are the subjects of highly regulated experiments. They can be adopted or ranked by quality. It’s in part our ability to manipulate and encounter them in this abundance of novel contexts that has generated so many questions. The narrator of Marilynne Robinson’s “Housekeeping” says, “Of my conception I know only what you know of yours. It occurred in darkness and I was unconsenting.” But conception now regularly unfolds under a microscope, and what once grew or failed to grow unseen is out in the open, under examination, up for debate.
I’m now the parent of a toddler I love more than I could have imagined and in possession of six remaining embryos, frozen and waiting for my decision on their fate. Like many people who have gone through I.V.F., I have a complicated relationship with them and vacillate between wanting the finality of deciding and holding on to the possibility that they might one day, under circumstances yet unknown, come to life.
I am also a journalist who covers reproduction, and I’ve reported on places where embryos figure prominently in ways that the public may not yet realize. Scientists are doing human embryo research that could, for instance, help prevent miscarriages. Companies are pushing the boundaries of what kind of testing can be done on embryos in the name of optimizing future lives. Embryos are at the center of divorce cases that are part property dispute, part custody battle.
What kind of rules should govern this research? Should businesses that profit from embryos be subject to any kind of oversight? And how should the law speak of them? Wherever embryos appear, they bring with them serious ethical and intellectual questions about what meaning or place they hold in our society.
The political fights over abortion demand a great deal of attention. But embryos also demand and warrant a meaningful conversation about everything they represent: values, knowledge, family, religion, health, life, death and more. The boundaries of what we are doing with embryos are shifting quickly. Any attempt to shape the future of how we treat them has to engage with these questions now.
For as long as scientists have worked with embryos, they’ve faced ethical questions about where to draw the line: How long is too long to grow a human embryo for research purposes? Two weeks? Four weeks? Or somewhere beyond? Does it matter if that research may one day help prevent miscarriages or serious fetal anomalies?
For decades, scientists around the world have abided by one widely accepted rule: Embryos being grown for research may not be cultivated beyond the 14-day mark.
That cutoff was based partly on biology — 14 days is typically when an embryo develops a structure known as the primitive streak, a sign it will no longer turn into twins — and partly on a recognition of the power of simple guidelines. (As the philosopher who helped hone those guidelines put it, “Everyone can count up to 14.”) It was also, mostly, an abstraction. No one, it was thought, could grow an embryo in a lab for anywhere close to two weeks anyway.
In fertility clinics, embryos typically grow in petri dishes for three to five days before being transferred into a patient’s uterus (or cryogenic storage); it was widely thought that an embryo required this transfer to continue developing. One attempt was reported in 1984 to have grown two embryos to eight or nine days, but one of them then stuck to the petri dish and began sprouting outgrowths; the other “became degenerate,” the scientists wrote, by the 197-hour mark.
So it was with some excitement that embryo researchers worldwide read about a 2013 experiment by Magdalena Zernicka-Goetz and her team of Cambridge University biologists. Using a culture medium enriched with nutrients and hormones, the researchers succeeded on their first attempt in growing two human embryos, donated by I.V.F. patients, beyond what was previously thought possible. When a colleague called Dr. Zernicka-Goetz at home, where she and her family were making dinner together, to tell her that one of them was still growing on Day 11, she was so thrilled, she couldn’t go to sleep that night.
Magdalena Zernicka-Goetz changed what is possible around researching human embryos. Until she and her team at Cambridge University had a breakthrough in 2013, no one had grown an embryo to 13 days in a lab environment.
The room in her Cambridge lab devoted to embryo and stem cell research is laid out like a galley kitchen that a real estate agent might euphemistically describe as compact; it can accommodate just about two scientists at a time. The research Dr. Zernicka-Goetz and her team conducted in those modest quarters yielded insights that changed how we understand the earliest days of human existence.
They discovered that by Days 8 or 9, embryonic cells began organizing themselves while they form different types of cells that will eventually become the placenta, the yolk sac that nourishes the embryo and the embryo itself. They found that the way the embryonic cells move and communicate with one another is what drives the formation of organs. And Dr. Zernicka-Goetz and her colleagues watched the process unfold in real time.
“Having insight into those stages of development opens this sort of — I don’t want to call it a Pandora’s box,” Dr. Zernicka-Goetz said, pausing to look for the right metaphor in English. (She grew up in Warsaw, and Polish is her first language.) “You’ve uncovered something that you’ve never looked at, and it’s full of gems.”
As the clock ticked toward Day 14, the Cambridge team had to end its experiments in order to stay compliant with the law. The team removed the remaining embryos from the culture dish and fixed them in slides in order to create images of them and further examine them. Eventually, the samples deteriorated and were disposed of as medical waste.
Any gems that lie beyond the 14-day mark remain out of reach. And so, depending on your perspective, a rule that was once hypothetical has become either a restraint holding us back from understanding a critical stage of human life or a crucial check on the scientific impulse to push limits simply because we can.
The rule may be poised to change. In 2021 the International Society for Stem Cell Research, a nonprofit scientific body that sets widely adopted global research norms, proposed that, contingent on “broad public support” and legality in a given jurisdiction, “a specialized scientific and ethical oversight process could weigh” whether researchers would be permitted to grow embryos beyond 14 days. The new guidelines call, first and foremost, for “public conversations touching on the scientific significance as well as the societal and ethical issues raised by allowing such research.” In some countries, this conversation has already begun.
Being able to examine an embryo at 11 days, like the one above, helps scientists understand how embryos develop from a single cell into different tissues, yielding a deeper understanding of pregnancy and our earliest days on earth.
Marta Shahbazi/Zernicka-Goetz Lab
The scientific significance of the research is clear. The period between the 14th day, when research must end, and the 28th day, when scientists can turn to embryonic tissue obtained from miscarriages or abortions to study, is when many pregnancies fail. It is also when organs begin forming and conditions such as cardiac abnormalities and neural tube defects arise. Observing that period of embryo development, often referred to as the black box of pregnancy, could lead to interventions for these developmental disorders and countless other medical breakthroughs. The societal and ethical issues, however, are also easy to grasp. Even people who do not equate embryos with human beings may be unsettled by the idea of growing them in dishes to increasingly advanced stages for research purposes.
Perhaps, as Dr. Zernicka-Goetz mused, their scientific triumph had opened a Pandora’s box after all.
In its journey from the fertility clinic where it was created to Dr. Zernicka-Goetz’s lab, an embryo loses one meaning and gains another. Perhaps its meaning changed many years ago, when it went from being a chance at a much-wanted baby to a conundrum, after a family felt itself complete. Perhaps it took a year or two or seven for the parents to decide they were done paying annual storage fees and were ready to choose a more definitive fate for their remaining embryos.
Dr. Zernicka-Goetz, like most developmental biologists, initially avoided doing human embryo research. It is riddled with controversy, is difficult to get approved, requires extensive specialized training and relies on costly equipment. For decades, she worked largely with embryos from mice and rats.
But that changed in 2006, when she received an unsettling call. She was pregnant, and a prenatal test that sampled her placental tissue had found 25 percent of the cells were genetically abnormal.
Unlike a majority of expecting patients, though, who might have panicked at this news, Dr. Zernicka-Goetz was intimately familiar with embryonic development through her research with mice. Her mind cycled through potential explanations. She suspected the issue was developmental, rather than hereditary, because a majority of the cells were normal. She also knew a developing embryo is remarkably resilient: In one research project, she removed cells from rapidly dividing mouse embryos and showed they grow into normal adult mice. In another, she and a student found that for mouse embryos with chromosomal abnormalities, their abnormal cells could be outcompeted by normal cells, dying at a rate more than twice that of the healthy ones. Mouse embryos, in other words, are capable of self-repair.
As it turned out, Dr. Zernicka-Goetz’s embryo was, too. Her son Simon is now a loving teenager whose paintings decorate her Caltech office in Pasadena, Calif., where she runs a sparkling new laboratory. But the results of her pregnancy screening left her deeply shaken, setting her on a new line of inquiry into the origins of human development.
The glass wall at the Caltech lab holds ideas for future experiments.
Her colleagues and mentors initially discouraged her from attempting to culture embryos beyond the pre-implantation stage; it would be far too difficult, they told her. Even Dr. Zernicka-Goetz suspected that embryos required interactions with the uterine lining to continue growing.
The title of the paper that resulted from the research that evolved from her 2013 experiment hints, in scientific argot, at what is perhaps most remarkable about her team’s findings: In “Self-Organization of the Human Embryo in the Absence of Maternal Tissues,” published in 2016, she and her co-authors detailed how, even with no mom in sight, a human embryo is capable of ambling down its developmental path on autopilot, well past the point when it would have normally implanted in the uterus.
After Dr. Zernicka-Goetz and her team’s successful experiment, another group, led by Ali Brivanlou at Rockefeller University, cultured embryos to Day 14. There is still more to be discovered within the bounds of the 14-day rule, as Dr. Zernicka-Goetz acknowledges. But many in the scientific community are already anticipating that crucial breakthroughs — the discoveries that might teach us why some babies are born with developmental defects, why some organs fail to grow properly and what causes miscarriages later in pregnancy — await us on the other side.
When I.V.F. proved a success in 1978, much of the media attention was on the birth of Louise Brown, the world’s first test-tube baby. But some were already worried about the research that had led to that moment — and what might follow.
Seeking ways to allay public concern, Britain set up a committee in 1982 to study the looming ethical issues that accompanied this new technology. It was tasked with establishing a blueprint for the country’s regulatory regime for I.V.F., as well as for the embryo research that made it and other fertility care possible. Among the concerns at the time, said Mary Warnock, the philosopher who chaired the committee, was a belief that “there was something especially horrendous in deliberately creating a human being only then to deprive it of its chance of life by failing to place it in a human womb but instead throwing it down the sink.”
The Warnock Committee, as it came to be known, was composed of scientists, social workers, a theologian and lawyers, among others. Its members quickly concluded that arriving at a societal consensus on the moral status of an embryo or establishing what could be done to it for legal purposes was a nearly impossible task. But the alternative was to have no limits or legislation at all, and this, Ms. Warnock said, “nobody wants.”
And so, according to a recent book on the 14-day rule’s history by the sociologist Sarah Franklin and the legal scholar Emily Jackson, the committee latched onto certain recognizable biological developments to which moral significance could be ascribed. The formation of the primitive streak, for instance, could be viewed as the moment an embryo transforms from pure potential — might it be twins or even triplets? — into a unique individual. Anne McLaren, an eminent biologist and the other public face of the Warnock Committee, said of the primitive streak, “If I had to point to a stage and say, ‘This is when I began being me,’ I would think it would have to be here.”
This life stage, previously given little notice, suddenly formed the basis for a whole chain of ethical reasoning. The 14-day rule has gone on to become the “de facto global regulatory standard for human embryo research,” Dr. Franklin and Dr. Jackson wrote.
Tubes are filled with media that can be used to grow mouse and human embryos past the point when they would normally implant in the uterus. While the Caltech group works with both human embryos and embryo models, their research usually begins with experiments on mouse embryos.
Such research has always been a source of controversy, regardless of how far into development it takes place. It has also yielded important findings, some of them related to fertility — such as more effective methods of freezing embryos and improved I.V.F. protocols — and some of them, such as the derivation of stem cells, that have generated entirely new areas of scientific research. Stem cells, which are now used in cancer treatments, among other therapies, were originally obtained from donated human embryos and aborted fetuses.
Today a majority of I.V.F. cycles still end in failure, and approximately one-third of infertility cases are classified as unexplained. The push past 14 days is, in part, a bid to better understand what makes a pregnancy stick. But there are other important questions at play. As embryonic cells start to differentiate into the body’s major organs, how do stem cells become brain cells? How does the heart take shape? What determines the fate of cells that are chromosomally abnormal?
“In my conscience, I know there are great benefits in pushing past 14 days,” Dr. Brivanlou has said. “It may literally save lives in the next generation.”
But the bioethicist Ben Hurlbut suspects others welcome the guardrails, worried about what they might otherwise conjure. He is troubled by the rapid leap from “can” — the ability to culture embryos beyond 14 days — to “should,” without much due paid to the basis for the rule. “Almost instantly, you have a kind of discourse of the need to revise the limit,” he told me. “Why? In what other domain of life would we say, when it becomes possible to violate a rule, that the rule has to give way to the violation?” The 14-day rule has always been slightly arbitrary; a 21- or 28-day rule would be even more so. What, then, would be stopping us from going even further?
While ethicists debate the 14-day rule, Dr. Zernicka-Goetz’s team and other research groups around the world have been pursuing a parallel research track — embryo models built from stem cells — that would allow scientists to probe the earliest days of human life while sidestepping some of the ethical questions. Building the models also allows scientists to better understand the underlying mechanics of embryo development, compared with simply observing it through a microscope. Supported by nutrients and grown in a dish, the current models can grow for roughly 10 days in ways that variously mimic key aspects of the real thing. But these embryo doppelgängers have led to their own flurry of moral and legal questions. Are they simply engineered tissue, or are they closer to a genuine embryo, deserving of special consideration?
Project 2025 calls for a number of actions whose net effect would be to take human embryo research off the table. Embryo models like the ones that will be grown from mouse stem cells in these tubes would be a possible — but far less faithful — alternative.
Most scientists working on these models, including Dr. Zernicka-Goetz, say the chances are almost nonexistent that any of them, for now, could go on to become a baby. (Models like these are being developed by teams elsewhere in the United States and in Israel, Britain and China, among other places.) In any case, implanting one in a human uterus would be an ethical breach, prohibited in countries that regulate this type of research, and a scientific scandal of the highest order.
But from a research perspective, the most important question is that of fidelity: How similar are these models to the real thing? If they skip a few of the developmental steps that a natural embryo passes through, as the models currently do, can we trust whatever they do next? As a group of scientists and medical ethicists, writing in Nature Methods, argued, if it’s not permissible in many countries to create egg-and-sperm embryos specifically for research purposes, why is it ethically preferable to create stem-cell-derived models that will become increasingly similar to natural embryos? And how can we assess their faithfulness to real embryos after 14 days without continuing to learn about the development of natural embryos anyway?
In light of these advances, an ethicist and several biologists have proposed a new legal definition for “embryo” that emphasizes its potential to become a fetus rather than whether it originated via fertilization or it was created in a lab from stem cells. With embryo models growing in sophistication, they argued in a commentary in the journal Cell, the relevant question is not how they got here but where they are going. A redefinition may offer some conceptual clarity, but it highlights the oddness of this moment — one in which our scientific prowess has pushed boundaries so far that we need to reconceive of entities whose previous technical definition seemed self-evident.
Today, Dr. Zernicka-Goetz’s lab at Caltech produces hundreds of embryo models every week. A vial the size of a pen cap holds several hundred of these embryo-like organisms, which look like minuscule air bubbles suspended in clear liquid.
On a warm September morning when I visited the facility, the sunny, open laboratory was still quiet, its chairs mostly empty, lab coats slung over the seat backs. The glass wall of the lab was covered in scribbled equations and diagrams that to an untrained eye look like the gibberish that teachers or geniuses hurriedly scrawl on blackboards in movies.
Mouse stem cells are stored at -80 degrees Celsius (-112 degrees Fahrenheit).
To speak to the two dozen or so scientists who work in the lab is to see how people working at the boundary of life navigate the uncanny valleys in which they sometimes find themselves. I met with Ikbal Choudhury, a postdoctoral scholar developing technology to create womblike tissue. In his experiments, when the embryo models’ cells communicate with one another in the course of their self-assembly, they produce the same hormones and chemical signals a natural embryo would, including human chorionic gonadotropin, or hCG. This is the same substance people test for when they’re wondering if they’re pregnant: I vividly recall taking an hCG blood test 13 days after my I.V.F.-generated embryo was transferred and learning, to my delight, that the embryo had indeed hung on. Dr. Choudhury told me that when the pre-implantation embryo models produce hCG, he and his colleagues joke that “the device is pregnant.”
I also met Sergi Junyent, a postdoctoral scholar from Spain, who was working with zygotes — that is, embryos created by the union of egg and sperm that have not yet undergone cell division.
His research involved waiting until each zygote had cleaved into two cells, then injecting one of the cells with a substance to turn its membrane fluorescent green, enabling him and his colleagues to trace that cell’s descendants. They then watched the dividing process unfold to see just how much of an embryo’s inner cell mass — the part that would become a future fetus rather than a placenta or yolk sac — came from only one of those two cells.
The results have given him pause. Dr. Junyent worked with stem cells and mouse embryos earlier in his career, but this was different. Each of his zygotes was identified by number, and he could tell you what each one was doing under the microscope at any given moment. He and his co-author, he said, “kind of lived for those embryos for the seven days the experiment lasted.”
Dr. Junyent and his wife were expecting their first child while the experiment was underway. It was “crazy,” he said, to think that the fertilization of his son — and the subsequent process he was witnessing under the microscope — occurred just a month and a half before. “At the two-cell stage, we already know what cell is more likely to become part of the embryo and to form body tissues.” Watching this process unfold from its earliest stages hasn’t affected his feelings about abortion law, exactly, which he thinks about primarily in terms of women’s bodily freedom. “But is this an embryo? Is this a future baby? Is this a single cell?” Dr. Junyent mused. “Or maybe it doesn’t matter that much.”
Ikbal Choudhury, pointing to an image of an embryo model, hopes for deeper public engagement in his research. The discoveries he and other scientists are making about the nature of human reproduction, he said, “will transform everyone.”
Whether they work with embryo models or human embryos, all of the scientists I spoke with were thinking deeply about the ethical implications of their research. But some have been genuinely troubled by them. Jianping Fu is a bioengineer at the University of Michigan who was part of a team that created one of the first stem-cell-based embryo models in 2017. In his work, he has backed away from creating what are known as integrated embryo models — the types that generate the supportive tissues necessary for implantation, such as those used by Dr. Choudhury. Dr. Fu focuses instead on simpler models that narrowly mimic a targeted aspect of embryo development. “I think there’s just a lot of ethical sensitivities, and personally I’m not OK with some of the integrated models,” he told me.
I found it disturbing to learn that while the existing methods of creating embryo models can generate hundreds of models at a time, not all of them develop into useful models; some self-organize into what one researcher described as “alternative arrangements.” As of now, the ones that do not self-organize into an embryo-like entity die off or are discarded. But what unexpected shape might they one day take? I was also not sure that my disquiet was entirely rational: Why was I so much more troubled by the alternative arrangements of these clumps of human cells than by the fate of, say, the multitude of skin cells I shed each day?
Alta Charo, a bioethicist who served on a national bioethics commission convened by President Bill Clinton that studied the issue of cloning, said such discomfort is to be expected in the wake of scientific advances. “As a species, we have experienced throughout our history many phenomena that felt like they were completely outside our control. And each time we manage to take control of them, I think there is a feeling of both awe and unease,” she said. But those feelings, Ms. Charo says, should not form the basis of policy.
The bioethicist Leon Kass, however, has argued that such unease is our moral intuition speaking and, as such, is worth heeding. After the cloning of Dolly the sheep in 1996, Dr. Kass argued in his essay “The Wisdom of Repugnance” that certain prospects — incest, cannibalism and, he would add, human cloning — inspire a revulsion that needs no rationalization. This repugnance, he writes, is a sign that “we intuit and feel, immediately and without argument, the violation of things we rightfully hold dear.”
It is too early to tell how President Trump will govern embryo research in his second term. It took until two years into his first administration for the Department of Health and Human Services to address the issue, but its actions were severe: It cut nearly all of its spending on research involving human fetal tissue. To add an extra layer of review for any projects that involved such tissue, the agency appointed an ethics review board whose members included many outspoken opponents of abortion; the board ultimately rejected 13 out of 14 proposals that had already been recommended for federal funding through the normal competitive process. The agency put out a call for alternatives to fetal tissue research, but the kinds of embryo models that Dr. Zernicka-Goetz and others make using stem cells were explicitly excluded from consideration.
For Dr. Zernicka-Goetz, an abnormal test result while she was pregnant with her son Simon set her on a new line of inquiry into the origins of human development.
This time, Mr. Trump stands between two constituencies. The conservative agenda for his second term known as Project 2025 calls for a number of actions whose net effect would be to take human embryo research off the table. Instead, it recommends pursuing alternative methods of research to make it so that “abortion and embryo-destructive related research, cell lines and other testing methods become both fully obsolete and ethically unthinkable.” The same influential evangelicals who have opposed research involving human embryos going back to George W. Bush’s administration more recently publicly stated their opposition to I.V.F. at last summer’s Southern Baptist Convention. After the Dobbs decision, a podcast hosted by Lila Rose, a leading anti-abortion activist, featured a guest decrying I.V.F. as a greater threat to embryonic life than abortion. “You’re more likely to have your life snuffed out by the people claiming to bring new life into the world than you are at Planned Parenthood,” she said.
But Mr. Trump is also surrounded by vocal natalists such as his donor and adviser Elon Musk, a prolific I.V.F. user, as well as Peter Thiel, who has invested in multiple fertility-related companies. It seems clear which side has won — for now. In February the president signed an executive order that calls for policy recommendations to protect access to I.V.F. and lower its costs (“because we want more babies,” Mr. Trump said in an accompanying statement).
The administration has a more limited ability to affect the 14-day rule, which in the United States isn’t a law but a guideline. Embryo research and research on fetal tissue are permitted on a state-by-state basis — New York, for example, has no limit on how long embryos may be grown for research purposes, whereas in California the guideline is 12 days — mirroring in some ways the current state of abortion access. The government has imposed restrictions through its federal funding, but much of the research on early human development relies on private funding from wealthy donors, foundations or venture capitalists.
So far, the issue has not yet garnered much public attention. It is possible this lack of awareness reflects the way that abortion — which has always been a proxy for a thousand other questions about gender, social roles and autonomy — has sucked almost all the air out of the room.
But pushing the frontiers of scientific research demands a broader discussion that transcends niche communities, especially in an area that could transform the nature of reproduction.
Public engagement is even more urgent at a time of historic mistrust in science, institutions and politics as a means of reaching consensus. The perils of pursuing research that triggers what ethicists call the ick factor without robust discussion risks generating further mistrust or even conspiracy theories.
In our recent past, there were times we pushed the boundaries of what science can do and then backed off, finding the ick factor too strong. The CRISPR-Cas9 technology, for example, which gives scientists the ability to edit the human genome, was discovered in 2012; it has been used to treat adult patients with sickle cell anemia, and researchers are hoping to use it to engineer crops that can withstand climate change. But its use on embryos has been the subject of a de facto global moratorium, as scientists are concerned about making changes to human DNA that has been passed down through millenniums. (The one exception: a scientist who claimed to have used it on three embryos, now young girls, in China — and subsequently faced backlash and prison time.) Similarly, the cloning of Dolly the sheep led to bans on human cloning and a self-imposed scientific moratorium, even though some hoped that reproductive cloning could generate a source of genetically matched stem cells to develop treatments for individual patients with devastating diseases.
On other occasions, the benefits seemed worth the trade-offs: Genetically modified food was embraced in the United States after initial concern about its effects on consumers; embryonic stem-cell research eventually proceeded in the 2000s after opposition from Mr. Bush, among others. These debates provided a forum for bigger societal questions. They helped people think through the trade-offs — what benefits could be in store even if the cost was some ick. They allowed people to feel they had a meaningful stake in a discussion about a technology that they might encounter personally and reassured them that the people developing these technologies were doing so out in the open.
All of the scientists I spoke to were eager for such a conversation to unfold around their work with human embryos. They understand the gravity of their work — its potential and the discomfort it could raise. Nicolas Rivron is the leader of a lab that produced one of the early stem-cell-based models and one of the scientists who proposed redefining “embryo.” He pointed to stories going back thousands of years to explain our discomfort with a power that seems godlike: Sumerian stories about creating people by mixing blood and clay, the Jewish tale of the golem, “Frankenstein” and “Brave New World.” Throughout history, he said, “You find myths and legends about humans making humans from scratch.” All of that places an obligation on scientists, he said, “from a very early, very early stage, to think about the potential intended and unintended consequences of our actions.” That is true. But it also puts an obligation on us.
Look for the second chapter of this series when it publishes next week.
Anna Louie Sussman is a journalist who writes about gender, economics and reproduction. This story was supported by the Pulitzer Center and the Alicia Patterson Foundation.
Embryo video via Geri Time-Lapse Camera/Clínica Fertility Madrid and Anna Louie Sussman.
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips. And here’s our email: letters@nytimes.com.
Follow the New York Times Opinion section on Facebook, Instagram, TikTok, Bluesky, WhatsApp and Threads.