Know-how Reporter
 Sierra Area
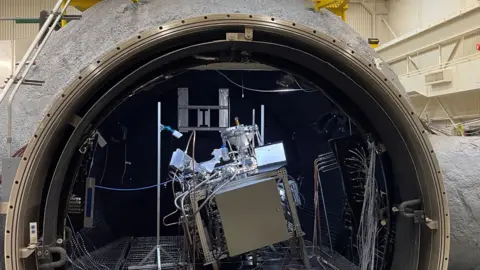
Sierra AreaInside an enormous sphere, the engineers pored over their gear. Earlier than them stood a silvery metallic contraption swathed in vibrant wires – a field that they hope will in the future make oxygen on the moon.
As soon as the workforce vacated the sphere, the experiment started. The box-like machine was now ingesting small portions of a dusty regolith – a combination of mud and sharp grit with a chemical composition mimicking actual lunar soil.
Quickly, that regolith was gloop. A layer of it heated to temperatures above 1,650C. And, with the addition of some reactants, oxygen-containing molecules started to bubble out.
“We’ve examined every part we are able to on Earth now,” says Brant White, a program supervisor at Sierra Area, a personal firm. “The following step goes to the moon.”
Sierra Area’s experiment unfolded at Nasa’s Johnson Area Heart this summer time. It’s removed from the one such expertise that researchers are engaged on, as they develop methods that would provide astronauts residing on a future lunar base.
These astronauts will want oxygen to breathe but in addition to make rocket gasoline for spacecraft that may launch from the moon and head to locations additional afield – together with Mars.
Lunar base inhabitants may also require metallic they usually might even harvest this from the dusty gray particles that litters the lunar floor.
A lot is determined by whether or not we are able to construct reactors capable of extract such assets successfully or not.
“It might save billions of {dollars} from mission prices,” says Mr White as he explains that the choice – bringing a number of oxygen and spare metallic to the moon from Earth – could be arduous and costly.
 Sierra Area
Sierra AreaFortunately, the lunar regolith is stuffed with metallic oxides. However whereas the science of extracting oxygen from metallic oxides, for instance, is properly understood on Earth, doing this on the moon is way more durable. Not least due to the situations.
The large spherical chamber that hosted Sierra Area’s checks in July and August this yr induced a vacuum and in addition simulated lunar temperatures and pressures.
The corporate says it has had to enhance how the machine works over time in order that it may possibly higher deal with the extraordinarily jagged, abrasive texture of the regolith itself. “It will get in every single place, wears out all kinds of mechanisms,” says Mr White.
And the one, essential, factor that you may’t check on Earth and even in orbit round our planet, is lunar gravity – which is roughly one sixth that of the Earth. It may not be till 2028 or later that Sierra Area can check its system on the moon, utilizing actual regolith in low gravity situations.
 NASA
NASAThe moon’s gravity may very well be an actual drawback for some oxygen-extracting applied sciences until engineers design for it, says Paul Burke at Johns Hopkins College.
In April, he and colleagues published a paper detailing the outcomes of laptop simulations that confirmed how a special oxygen-extracting course of may be hindered by the moon’s comparatively feeble gravitational pull. The method beneath investigation right here was molten regolith electrolysis, which entails utilizing electrical energy to separate lunar minerals containing oxygen, in an effort to extract the oxygen immediately.
The issue is that such expertise works by forming bubbles of oxygen on the floor of electrodes deep inside the molten regolith itself. “It’s the consistency of, say, honey. It is rather, very viscous,” says Dr Burke.
“These bubbles aren’t going to rise as quick – and may very well be delayed from detaching from the electrodes.”
There may very well be methods round this. One may very well be to vibrate the oxygen-making machine gadget, which could jiggle the bubbles free.
And further-smooth electrodes would possibly make it simpler for the oxygen bubbles to detach. Dr Burke and his colleagues are actually engaged on concepts like this.
Sierra Area’s expertise, a carbothermal course of, is totally different. Of their case, when oxygen-containing bubbles kind within the regolith, they achieve this freely, quite than on the floor of an electrode. It means there’s much less probability of them getting caught, says Mr White.
Stressing the worth of oxygen for future lunar expeditions, Dr Burke estimates that, per day, an astronaut would require the quantity of oxygen contained in roughly two or three kilograms of regolith, relying on that astronaut’s health and exercise ranges.
Nonetheless, a lunar base’s life assist methods would probably recycle oxygen breathed out by astronauts. If that’s the case, it wouldn’t be essential to course of fairly as a lot regolith simply to maintain the lunar residents alive.
The actual use case for oxygen-extracting applied sciences, provides Dr Burke, is in offering the oxidiser for rocket fuels, which might allow bold area exploration.
 MIT and Shaan Jagani
MIT and Shaan JaganiClearly the extra assets that may be made on the moon the higher.
Sierra Area’s system does require the addition of some carbon, although the agency says it may possibly recycle most of this after every oxygen-producing cycle.
Together with colleagues, Palak Patel, a PhD scholar on the Massachusetts Institute of Know-how, got here up with an experimental molten regolith electrolysis system, for extracting oxygen and metallic from the lunar soil.
“We’re actually it from the standpoint of, ‘Let’s attempt to minimise the variety of resupply missions’,” she says.
When designing their system, Ms Patel and her colleagues addressed the issue described by Dr Burke: that low gravity might impede the detachment of oxygen bubbles that kind on electrodes. To counter this, they used a “sonicator”, which blasts the bubbles with sound waves in an effort to dislodge them.
Ms Patel says that future resource-extracting machines on the moon might derive iron, titanium or lithium from regolith, for instance. These supplies would possibly assist lunar-dwelling astronauts make 3D-printed spare elements for his or her moon base or alternative elements for broken spacecraft.
The usefulness of lunar regolith doesn’t cease there. Ms Patel notes that, in separate experiments, she has melted simulated regolith into a tricky, darkish, glass-like materials.
She and colleagues labored out the way to flip this substance into sturdy, hole bricks, which may very well be helpful for constructing constructions on the moon – an imposing black monolith, say. Why not?

